New lithium battery uses waste graphite
October 17, 2017
on
on
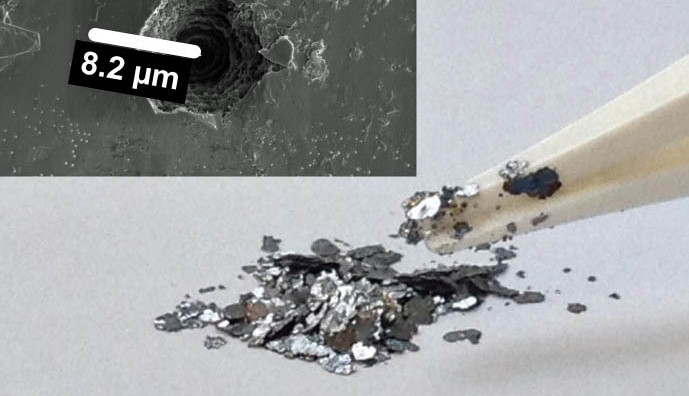
Researchers from the EMPA (Swiss Federal Material Testing and Research Institute) together with experts from ETH Zurich have developed a new design of rechargeable battery made from waste graphite and recycled metals. For this design they turn the conventional lithium-ion battery structure on its head. This new construction is said to be more cost-effective and according to the initial tests, also more durable.
In conventional lithium-ion battery design the negative electrode (anode) is made from graphite, this new design however uses graphite as the positive electrode (cathode). When the battery is in a charged state the organic anions, which are larger than metal ions, are deposited in the intermediate spaces in the graphite. In this new battery, the anode is made of metal.
Natural graphite is suitable when it is in the form of coarse flakes and not too finely ground. The reason for this is that the graphite flakes are open at the edges and the relatively thick anions can easily penetrate into the structure. Fine-grained graphite, used in lithium-ion batteries, is not suitable for this new concept: when the graphite particles are finely ground, the layers become creased like crumpled paper. Only small lithium ions are able to penetrate this crumpled graphite, not the thicker anions employed in the structure of this new battery.
In conventional lithium-ion battery design the negative electrode (anode) is made from graphite, this new design however uses graphite as the positive electrode (cathode). When the battery is in a charged state the organic anions, which are larger than metal ions, are deposited in the intermediate spaces in the graphite. In this new battery, the anode is made of metal.
Natural graphite is suitable when it is in the form of coarse flakes and not too finely ground. The reason for this is that the graphite flakes are open at the edges and the relatively thick anions can easily penetrate into the structure. Fine-grained graphite, used in lithium-ion batteries, is not suitable for this new concept: when the graphite particles are finely ground, the layers become creased like crumpled paper. Only small lithium ions are able to penetrate this crumpled graphite, not the thicker anions employed in the structure of this new battery.
Read full article
Hide full article


Discussion (0 comments)