Hybrid solar system makes rooftop hydrogen
on
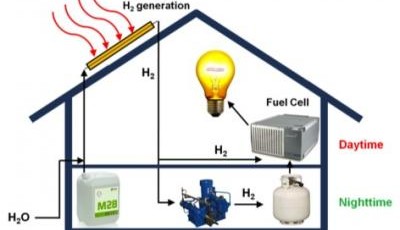
While roofs across the world sport photovoltaic solar panels to convert sunlight into electricity, a
Instead of systems based on standard solar panels,
For his analysis, Hotz compared the hybrid system to three different technologies in terms of their exergetic performance. Exergy is a way of describing how much of a given quantity of energy can theoretically be converted to useful work.
"The hybrid system achieved exergetic efficiencies of 28.5 percent in the summer and 18.5 percent in the winter, compared to 5 to 15 percent for the conventional systems in the summer, and 2.5 to 5 percent in the winter," said Hotz, assistant professor of mechanical engineering and materials science at Duke’s Pratt School of Engineering.
The paper describing the results of Hotz's analysis was named the top paper during the ASME Energy Sustainability Fuel Cell 2011 conference in Washington, D.C. Hotz recently joined the Duke faculty after completing post-graduate work at the University of California-Berkeley, where he analyzed a model of the new system. He is currently constructing one of the systems at Duke to test whether or not the theoretical efficiencies are born out experimentally.
Hotz's comparisons took place during the months of July and February in order to measure each system’s performance during summer and winter months.
Like other solar-based systems, the hybrid system begins with the collection of sunlight. Then things get different. While the hybrid device might look like a traditional solar collector from the distance, it is actually a series of copper tubes coated with a thin layer of aluminum and aluminum oxide and partly filled with catalytic nanoparticles. A combination of water and methanol flows through the tubes, which are sealed in a vacuum.
The three systems examined in the analysis were the standard photovoltaic cell which converts sunlight directly into electricity to then split water electrolytically into hydrogen and oxygen; a photocatalytic system producing hydrogen similar to Hotz's system, but simpler and not mature yet; and a system in which photovoltaic cells turn sunlight into electricity which is then stored in different types of batteries (with lithium ion being the most efficient).
Costs and efficiencies of systems can vary widely depending on location – since the roof-mounted collectors that could provide all the building's needs in summer might not be enough for winter. A rooftop system large enough to supply all of a winter’s electrical needs would produce more energy than needed in summer, so the owner could decide to shut down portions of the rooftop structure or, if possible, sell excess energy back to the grid.
Hotz’s research was supported by the Swiss National Science Fund. Joining him in the study were UC-Berkeley's Heng Pan and Costas Grigoropoulos, as well as Seung H. Ko of the Korea Advanced Institute of Science and Technology, Daejon.
Source: Richard Merritt


Discussion (0 comments)