New Battery Technology Charges 1000 Times Faster
on
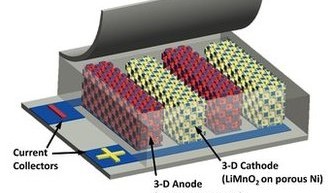
A team at the University of Illinois has unveiled a battery design which offers 10 times the energy density and 1000 times faster recharge time compared to current cell technology according to a paper in the Journal Nature Communications.
The battery uses a LiMnO2 cathode and NiSn anode but the real innovation is in the novel electrode design. The electrodes are fabricated using a lattice of tiny polystyrene spheres which are coated with metal. The spheres are then dissolved to leave a 3D-metal scaffold onto which a nickel-tin alloy is added to form the anode, and the mineral manganese oxyhydroxide forms the cathode. In the last stage the glass surface is immersed into a liquid heated to 300˚C (572˚F).
The resulting structure massively increases the electrode surface area and reduces the clearance between the electrodes. The resulting smaller gap means that the electrons and ions travel a shorter distance allowing faster access to the stored energy.


Discussion (0 comments)