Research Points the way to Safer Lithium Batteries
June 29, 2015
on
on
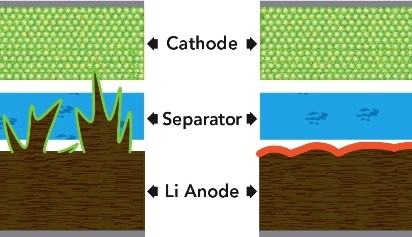
A paper published in the June 17th edition of Nature Communications describes how the addition of two chemicals to the electrolyte of lithium metal batteries can prevent the formation of dendrites. These are needles of lithium which grow in the battery and eventually puncture the barrier between the two battery halves. Their formation can cause short circuits in the battery which leads to overheating and sometimes combustion.
According to the paper this breakthrough could help remove a major barrier to the future development of lithium-sulfur and lithium-air batteries. These promising new battery technologies could store up to 10 times more energy per weight than batteries in use today in consumer electronics and electric cars.
Yi Cui, an associate professor at Stanford University and the US Department of Energy’s SLAC National Accelerator Laboratory said that one of the compounds added to the electrolyte, lithium nitrate, has been under investigation for a long time as an additive to improve battery performance. The other, lithium polysulfide, has been considered a nuisance: Formed when a sulfur electrode degrades, it travels to the lithium metal electrode and wrecks it.
The team realized that the combined effect of these two compounds had not been studied before; together the chemicals could potentially react with lithium metal to form a stable, solid interface between the electrode and the electrolyte. They assembled coin cell batteries and added various concentrations of the two chemicals to the ether-based electrolyte. After many charge/discharge cycles they took them apart and examined the electrodes with an electron microscope and an X-ray technique that reveals their morphology and chemical composition.
They found that adding both chemicals in just the right amounts stopped lithium dendrite formation; harmless pancake-like deposits grew instead. The lithium metal electrode acquired a stable coating that helped protect it from further degradation and actually improved the battery’s performance.
In tests, batteries with both chemicals added operated at 99 percent efficiency after more than 300 charge-discharge cycles, compared to significantly decreased efficiency after 150 cycles for batteries treated with lithium nitrate alone, said Fiona (Weiyang) Li, a postdoctoral researcher in Cui’s lab and first author of the paper.
Yet-Ming Chiang, a professor at the Massachusetts Institute of Technology, collaborated with the team and helped them interpret the results. He said the next step is to see if this approach can prevent dendrite formation in larger-scale cells that are closer to being practical batteries. It may also work for electrodes made of other metals, such as magnesium, calcium or aluminum, that also have potential for storing much more energy than today’s batteries. For more information read the press release.
According to the paper this breakthrough could help remove a major barrier to the future development of lithium-sulfur and lithium-air batteries. These promising new battery technologies could store up to 10 times more energy per weight than batteries in use today in consumer electronics and electric cars.
Yi Cui, an associate professor at Stanford University and the US Department of Energy’s SLAC National Accelerator Laboratory said that one of the compounds added to the electrolyte, lithium nitrate, has been under investigation for a long time as an additive to improve battery performance. The other, lithium polysulfide, has been considered a nuisance: Formed when a sulfur electrode degrades, it travels to the lithium metal electrode and wrecks it.
The team realized that the combined effect of these two compounds had not been studied before; together the chemicals could potentially react with lithium metal to form a stable, solid interface between the electrode and the electrolyte. They assembled coin cell batteries and added various concentrations of the two chemicals to the ether-based electrolyte. After many charge/discharge cycles they took them apart and examined the electrodes with an electron microscope and an X-ray technique that reveals their morphology and chemical composition.
They found that adding both chemicals in just the right amounts stopped lithium dendrite formation; harmless pancake-like deposits grew instead. The lithium metal electrode acquired a stable coating that helped protect it from further degradation and actually improved the battery’s performance.
In tests, batteries with both chemicals added operated at 99 percent efficiency after more than 300 charge-discharge cycles, compared to significantly decreased efficiency after 150 cycles for batteries treated with lithium nitrate alone, said Fiona (Weiyang) Li, a postdoctoral researcher in Cui’s lab and first author of the paper.
Yet-Ming Chiang, a professor at the Massachusetts Institute of Technology, collaborated with the team and helped them interpret the results. He said the next step is to see if this approach can prevent dendrite formation in larger-scale cells that are closer to being practical batteries. It may also work for electrodes made of other metals, such as magnesium, calcium or aluminum, that also have potential for storing much more energy than today’s batteries. For more information read the press release.
Read full article
Hide full article


Discussion (0 comments)