Waste blue dye could provide green solution
September 24, 2018
on
on
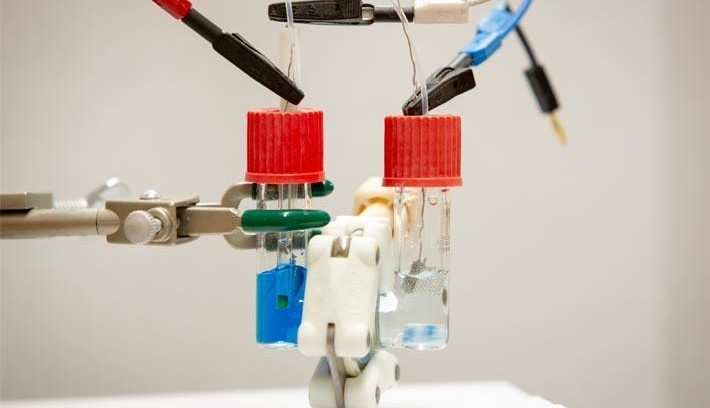
Wastewater from textile factories often contain the chemical methylene blue which is commonly used as a fabric dye. As well as its function as a dye this chemical is used in medicine to treat various conditions. Research is now indicating it may also be useful as an effective electrolyte material for redox flow batteries.
Methylene blue is a water-soluble powder used to dye paper and especially textiles such as denim. This colorant is toxic in the environment as is difficult to remove from waste water. Researchers at the University of Buffalo are exploring other uses for this chemical and succeeded in using waste methylene blue as an electrolyte in a redox flow battery design.
Redox flow batteries are in principle batteries in which the actual electrodes only need to be relatively small compared to the overall system reaction chamber, the electrolyte which contains the actual charge in the form of chemical energy is pumped between two tanks pass the electrodes. Tank 1 contains electrolyte in the low-energy state, tank 2 in high-energy state. During charging, current flows through the electrodes as the electrolyte is pumped between the tanks. When discharged, the electrolyte flows in reverse. The advantage of a flowing pumped electrolyte is that the electrode surface area does not need to be as large as those used in conventional battery design. Electrodes are usually some of the most expensive parts of the battery; smaller electrodes equals cheaper batteries. The flowing electrolyte is still able to store large amounts of energy. This technique is particularly interesting for battery installations which act as energy reservoirs for generators such as wind turbines where the energy produced can suffer from a high level of variability.
Methylene blue is difficult to remove from the dye factory effluent so this new application provides a win-win solution, large quantities of a cheap electrolyte will be available that would otherwise have to be disposed of with great difficulty. Salts are also used in the dyeing process and are combined with the methyl blue wastewater. Electrolytes for redox flow batteries need this salt anyway to support the electrolyte function so again the solution seems ideal. Initial experiments with this new battery design showed high stability over more than 50 charge cycles. The next step will be a real-world trial using dye factory waste water.
Methylene blue is a water-soluble powder used to dye paper and especially textiles such as denim. This colorant is toxic in the environment as is difficult to remove from waste water. Researchers at the University of Buffalo are exploring other uses for this chemical and succeeded in using waste methylene blue as an electrolyte in a redox flow battery design.
Redox flow batteries are in principle batteries in which the actual electrodes only need to be relatively small compared to the overall system reaction chamber, the electrolyte which contains the actual charge in the form of chemical energy is pumped between two tanks pass the electrodes. Tank 1 contains electrolyte in the low-energy state, tank 2 in high-energy state. During charging, current flows through the electrodes as the electrolyte is pumped between the tanks. When discharged, the electrolyte flows in reverse. The advantage of a flowing pumped electrolyte is that the electrode surface area does not need to be as large as those used in conventional battery design. Electrodes are usually some of the most expensive parts of the battery; smaller electrodes equals cheaper batteries. The flowing electrolyte is still able to store large amounts of energy. This technique is particularly interesting for battery installations which act as energy reservoirs for generators such as wind turbines where the energy produced can suffer from a high level of variability.
Methylene blue is difficult to remove from the dye factory effluent so this new application provides a win-win solution, large quantities of a cheap electrolyte will be available that would otherwise have to be disposed of with great difficulty. Salts are also used in the dyeing process and are combined with the methyl blue wastewater. Electrolytes for redox flow batteries need this salt anyway to support the electrolyte function so again the solution seems ideal. Initial experiments with this new battery design showed high stability over more than 50 charge cycles. The next step will be a real-world trial using dye factory waste water.
Read full article
Hide full article


Discussion (0 comments)