Decomposition of water using solar energy
on
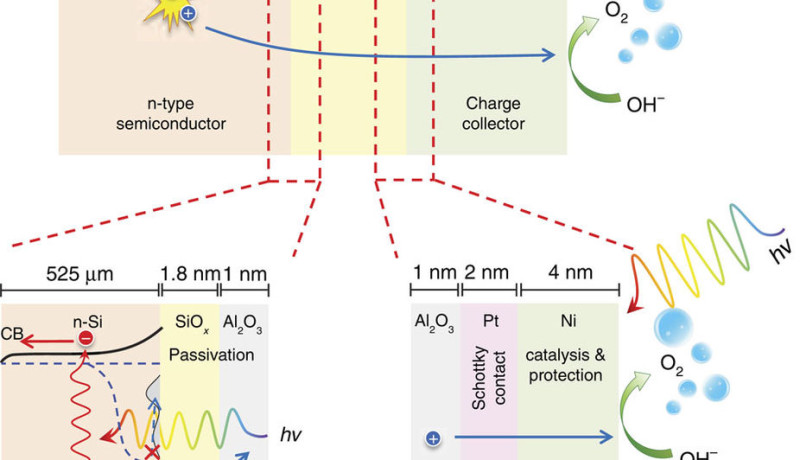
Decomposition of water using solar energy offers interesting possibilities for efficient large-scale conversion and storage of renewable energy. Researchers from the TU Delft and AMOLF have built an efficient and stable photo-electrode that absorbs light and directly decomposes water into hydrogen and oxygen. Silicon wafers are used for the light-absorbing material, so that the system is cheap as well. The researchers have already published their discoveries in Nature Communications.
Energy conversion
Photo-electrochemical (PEC) decomposition of water is seen as a sustainable method for the production of a clean and renewable fuel. With this, solar energy is directly converted into chemical energy. The hydrogen can, for example, be used in the fuel cells of electric cars, or be converted into other durable materials. The new photo-electrode is very efficient and remains stable for more than 200 hours. The latter is very special: usually no more than 5 hours of stability is obtained.
MIS
It is essential that a PEC system has a sufficiently large photo-current and photo-voltage to make the water oxidation reaction possible. There is usually a compromise between efficiency and stability: improving one inevitably results in the deterioration of the other. With the new electrode both these problems where considered separately and the results only combined at the end. This uses a new insulation layer to stabilize the silicon photo-electrode, and two metals to increase the photo voltage. This approach, in the shape of a metal-insulator-semiconductor junction (MIS) was already shown to be efficient, and is now also durable.


Discussion (1 comment)