Greenhouse effect reversed: fuel from CO2
February 04, 2016
on
on
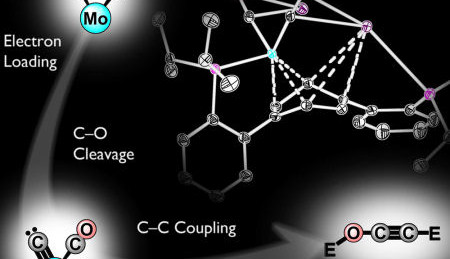
Reversing the greenhouse effect and at the same time accessing a new source of fuel? It may perhaps soon be possible, according to a study by Caltech, California Institute of Technology in Pasadena, California. Professor Theo Agapie and graduate Joshua Buss have developed a model which describes the initial steps of a process for converting CO2 to hydrocarbons.
Through photosynthesis plants convert sunlight, water and CO2 into sugars and carbon compounds which feeds the processes inside the cells. CO2 is therefore both an ingredient for the production of fossil fuels as well as a product of the combustion of the fuel. The Fischer–Tropsch process is a well-known process in which hydrogen gas (H2) and carbon monoxide (CO) are converted into fuel. The mechanism behind this is not yet fully understood however and the process requires very high pressures (up to 100 bar) and temperatures (up to 300 ºC).
In their study, Agapie and Buss have synthesized a new metallic compound based on Molybdenum, which makes the separation of the CO molecule easier (a method to convert CO2 into CO already exists). The C-O bonds are weakened and with the introduction of silyl ether -electrophile are broken completely. Finally the 2 CO-molecules are converted at room temperature into an ethynol derivative, where extracting the C2 products from the metal is the most important step.
The ethynol derivative is not usable as a fuel as such, but is a step in the direction of producing synthetic hydrocarbon based fuels.
The study is published in the 7 January 2016 edition ofNature. More information: http://authors.library.caltech.edu/63155.
Source: www.caltech.edu/news/toward-liquid-fuels-carbon-dioxide-49074.
Through photosynthesis plants convert sunlight, water and CO2 into sugars and carbon compounds which feeds the processes inside the cells. CO2 is therefore both an ingredient for the production of fossil fuels as well as a product of the combustion of the fuel. The Fischer–Tropsch process is a well-known process in which hydrogen gas (H2) and carbon monoxide (CO) are converted into fuel. The mechanism behind this is not yet fully understood however and the process requires very high pressures (up to 100 bar) and temperatures (up to 300 ºC).
In their study, Agapie and Buss have synthesized a new metallic compound based on Molybdenum, which makes the separation of the CO molecule easier (a method to convert CO2 into CO already exists). The C-O bonds are weakened and with the introduction of silyl ether -electrophile are broken completely. Finally the 2 CO-molecules are converted at room temperature into an ethynol derivative, where extracting the C2 products from the metal is the most important step.
The ethynol derivative is not usable as a fuel as such, but is a step in the direction of producing synthetic hydrocarbon based fuels.
The study is published in the 7 January 2016 edition ofNature. More information: http://authors.library.caltech.edu/63155.
Source: www.caltech.edu/news/toward-liquid-fuels-carbon-dioxide-49074.
Read full article
Hide full article


Discussion (0 comments)