Never wipe your glasses again
on
Nanostructure, fluorinated silica coating repels water and oil effectively
Eyeglasses need never again to be cleaned, and dirty windscreens are a thing of the past! Researchers at the Max Planck Institute for Polymer Research in
A transparent coating that’s very good at repelling water and oil, as is now being presented by the Mainz-based researchers, could not only keep water and dirt away from the lenses in glasses and car windscreens, but also, for example, from the glass facades of skyscrapers. It could also prevent residues of blood or contaminated liquids on medical equipment.
The coating essentially consists of an extremely simple material: silica, the main constituent of all glass. The researchers coated this with a fluorinated silicon compound, which already makes the surface water and oil repellent, like a non-stick frying pan. The really clever part is the structure of the coating, however. This is what makes the glass super water repellent and super oil repellent. In a frying pan with this type of coating, water and oil would simply roll around in the form of drops. The structure of the layer resembles a sponge-like labyrinth of completely unordered pores, which is made up of tiny spheres.
The researchers began by holding a glass slide in a flame so that the soot particles, which measure around 40 nanometers in diameter, formed a sponge-like structure on the glass. The next step was to coat it with silica in a glass vessel – even a jam jar would do – by vapor depositing a volatile organic silicon compound and ammonia onto the soot deposit. When they subsequently heated the material, the soot decomposed. The next step was to vapor deposit a fluorinated silicon compound as well onto the hollow silica structure.
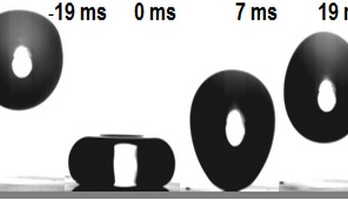
They then attempted to wet this coating with different liquids. However, they didn’t succeed, even when they let hexadecane drip from a great height onto it; in a non-stick frying pan, hexadecane spreads out like water in a washbasin. Although a portion of the liquid remained in the pores and wet the material, when most of the drop returned to the surface at a slower speed after bouncing up, it drew the small amount of the hexane that had remained out of the glass pores again. Finally, the reunited drop remained lying on the surface like a ball. The researchers in
Through the aid of the new coatings the researchers want to find out more about the factors that determine how well a material repels water and oil. As soon as the researchers have achieved a systematic understanding of why a liquid wets a surface or not, industrial companies will be able to specifically develop self-cleaning coatings for applications in architecture, optics and medicine.


Discussion (0 comments)