Photo-catalyst converts CO2 into CO
on
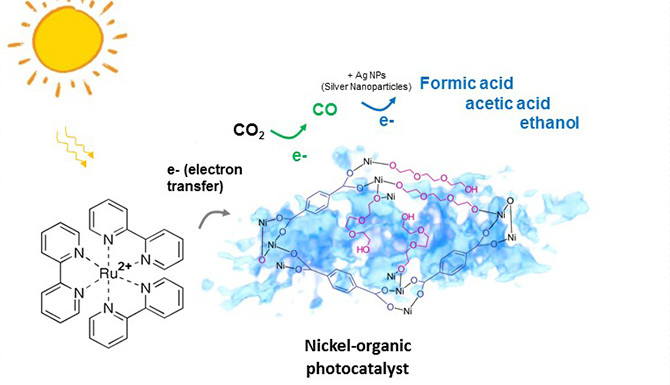
Researchers from the Lawrence Berkeley National Laboratory (California, United States) have developed a material that under the influence of light converts carbon dioxide (CO2) chemically into carbon monoxide (CO). By itself this is not really remarkable; however, the exceptional part is that this reaction does not create any undesirable byproducts. This discovery therefore means an important step in the direction of developing a technology that can produce fuels and other energy-dense compounds with the aid of a catalyst that operates from solar energy; at the same time this method would reduce the amount of CO2 in the atmosphere (one of the infamous greenhouse gases).
Selective reaction
The catalyst comprises a 'spongy' nickel/organic crystalline structure which converts CO2 inside a reaction chamber into CO, that forms the building block for liquid fuels (such as ethanol), solvents and other useful products.
The production of CO has a selectivity of nearly 100% - that means that no 'competing' gases such as hydrogen or methane are formed. And this achievement is a big step forwards. When reducing carbon dioxide to carbon monoxide the goal is, after all, to obtain only a single end product and not a mixture of different compounds that then have to be separated with much effort.
CO2 reduction to CO
The new catalyst material has been tested in a reaction chamber that is filled with carbon dioxide. The reaction products were determined with the aid of gas chromatography and mass spectrometry. It turns out that 1 gram of catalyst in 1 hour at room temperature can produce about 400 ml of CO; in addition it appears that the catalyst remains stable for a longer period of time.


Discussion (0 comments)