Replicate surface structures at atomic scale
on
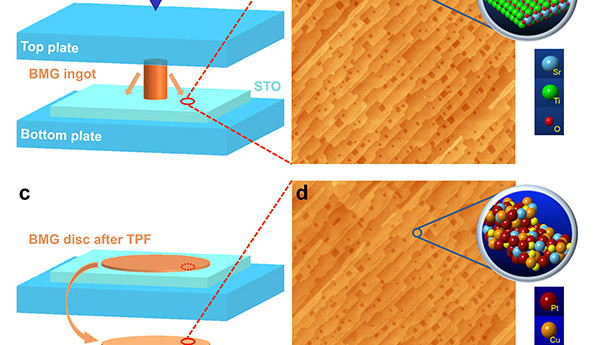
Researchers from Yale University (United States) have developed a procedure that can replicate surface structures down to the smallest detail (at atomic scale). This breakthrough could lead to better catalysts, better storage media and countless new applications.
Not just once
The researchers, under the direction of professor Udo Schwarz, have succeeded multiple times, and in a reliable manner, in replicating the 'terraced' surface of strontium titanate crystals (SrTiO3) . These crystals act like a ‘mould’; using a so-called amorphous metal (’metallic glass’), an impression was made.
The results of the research are published in Communications Physics.
Amorphous metal
The process involves (among other things) heating a metallic glass, which mostly consists of platinum, that is subsequently pressed into the mould. This is comparable to the techniques that are used to mass manufacture plastic toys and enclosures ― but then at an unimaginably smaller scale. Most metals have a crystalline structure, that means that the atoms of a metal are arranged in a grid. In contrast, in an amorphous metal (usually this is an alloy) the atoms or not ordered ― each atom can move independently of the others once the temperature is reached that changes the metal into a glass-like mass.


Discussion (0 comments)