Thin layer in Li-ion battery holds the key
April 25, 2018
on
on
Researchers working at the American Argonne National Laboratory have been able to shed more light on the chemical processes active in lithium batteries which determine the cell’s durability and stability. The relevant reactions occur in a microscopically thin layer called the ‘solid-electrolyte interphase’ or SEI which forms in the battery at the interface between the fluid electrolyte and the solid electrode.
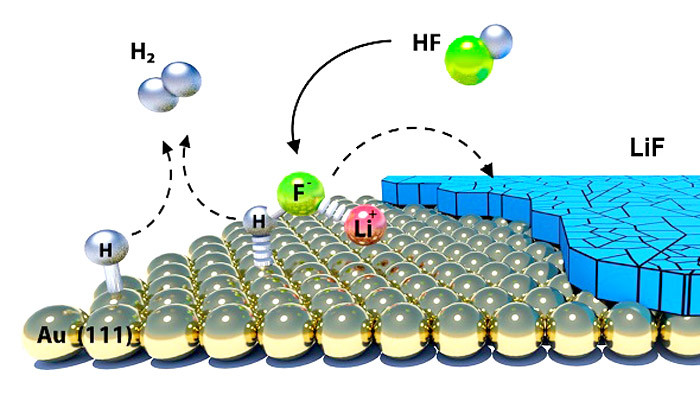
When charging a lithium battery, the electrochemical reaction of hydrogen fluoride produces the compound lithium fluoride, which significantly affects the performance of a battery. The reaction is highly dependent on the material the negative electrode is made of; it could be a metal, graphene or graphitic material. The choice of this material is crucial as it determines the nature and extent of the chemical reactions. The team used a novel method to monitor the concentration of hydrogen fluoride which is a detrimental impurity produced in the reaction between a salt in the electrolyte and traces of moisture. This new monitoring method will be an important tool to help facilitate further studies of the SEI.
More details can be found in the Argonne press release: Battery’s hidden layer revealed.
When charging a lithium battery, the electrochemical reaction of hydrogen fluoride produces the compound lithium fluoride, which significantly affects the performance of a battery. The reaction is highly dependent on the material the negative electrode is made of; it could be a metal, graphene or graphitic material. The choice of this material is crucial as it determines the nature and extent of the chemical reactions. The team used a novel method to monitor the concentration of hydrogen fluoride which is a detrimental impurity produced in the reaction between a salt in the electrolyte and traces of moisture. This new monitoring method will be an important tool to help facilitate further studies of the SEI.
Improved Li-ion battery design
Since initial research into practical lithium-ion cells back in the 1970s at the Technical University of Munich, attempts have been made to explain in detail the processes occurring at the SEI and the precise composition of this 1 μm layer. The battery characteristics are largely dependent on the quality of the SEI. Now that these processes are better understood there will be opportunities to further optimize Li-ion battery design and improve durability. The research results have already seen successful application in some commercial battery designs.More details can be found in the Argonne press release: Battery’s hidden layer revealed.
Read full article
Hide full article


Discussion (0 comments)