Lithium-ion cells with solid electrolyte promises fast charging
February 28, 2018
on
on
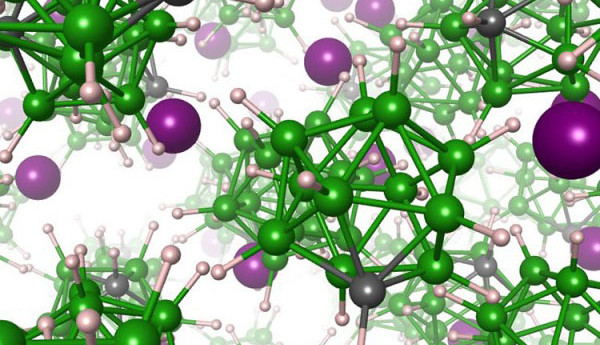
Research in the design of lithium-ion batteries using solid-state electrolyte material is still in its infancy. This new type of battery using non flammable materials promises better safety and improved energy density compared to current Li-ion batteries with fluidic electrolyte. A major hurdle in the development so far has been the search for a solid state electrolyte with the right properties, allowing fast transfer of lithium ions between the electrodes.
Other problem areas of solid state electrolyte relate to the long-term stability and durability of the battery. In order to promote speedier passage of lithium ions it’s been necessary to make the electrolyte as thin as possible but this impacts on overall battery durability. Researchers at the Lawrence Livermore National Laboratory and the National Institute of Standards and Technology have made a significant advance using a new class of materials called closo-borates which offer fast lithium ion mobility. By substituting one boron atom for a carbon atom in this key electrolyte material they found that the lithium ions moved around faster and facilitated faster charging.
This new electrolyte material is a salt made up of negatively charged closo-borate anions and positively charged lithium cations. The closo-borate anions quickly align themselves, spinning around in the solid matrix as they switch between preferred alignments.
Adding carbon to the closo-borate anion produces a dipole, which repels lithium around the carbon atom. As the anion turns, the carbon atom is aligned in different planes, forcing lithium to move away to a neighboring site in the solid matrix. The salt is therefore a mass of rotating anions which facilitates very rapid movement of lithium. Research is ongoing to further refine this new electrolyte material.
Other problem areas of solid state electrolyte relate to the long-term stability and durability of the battery. In order to promote speedier passage of lithium ions it’s been necessary to make the electrolyte as thin as possible but this impacts on overall battery durability. Researchers at the Lawrence Livermore National Laboratory and the National Institute of Standards and Technology have made a significant advance using a new class of materials called closo-borates which offer fast lithium ion mobility. By substituting one boron atom for a carbon atom in this key electrolyte material they found that the lithium ions moved around faster and facilitated faster charging.
This new electrolyte material is a salt made up of negatively charged closo-borate anions and positively charged lithium cations. The closo-borate anions quickly align themselves, spinning around in the solid matrix as they switch between preferred alignments.
Adding carbon to the closo-borate anion produces a dipole, which repels lithium around the carbon atom. As the anion turns, the carbon atom is aligned in different planes, forcing lithium to move away to a neighboring site in the solid matrix. The salt is therefore a mass of rotating anions which facilitates very rapid movement of lithium. Research is ongoing to further refine this new electrolyte material.
Read full article
Hide full article


Discussion (0 comments)