New ultrathin semiconductors beat silicon
August 29, 2017
on
on
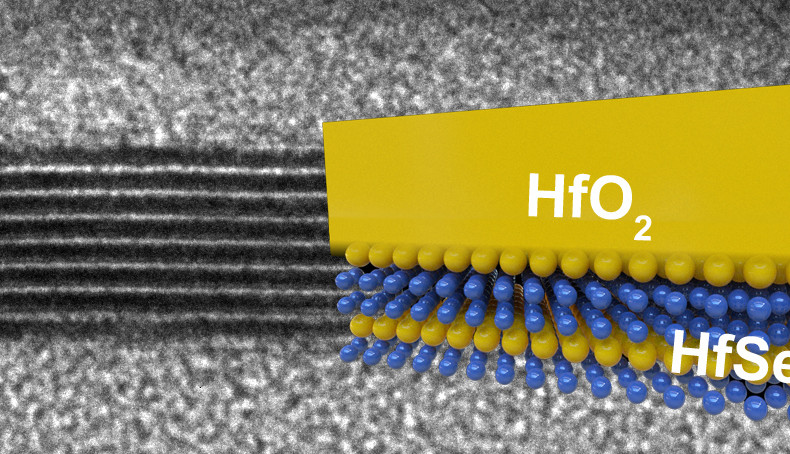
Silicon dioxide is a good insulator, and can be obtained by exposing silicon to oxygen. Chip manufacturers make use of this property to isolate their circuits on a die. Other semiconductors do not have this desirable property and require additional insulating layers, which is why today silicon is still the main material for building chips. But this may change in the future now that researchers at Stanford have identified two semiconductor materials that, when oxidized, become insulators, and good ones too.
The two materials—hafnium diselenide and zirconium diselenide— “rust” even better than silicon as they form so-called high-K insulators. Because of this they can be used in thinner layers, enabling lower power operation than is possible with silicon. Furthermore, these ultrathin semiconductors feature a band gap (an energy range in a solid where no electron states can exist) similar to silicon, giving them similar switching properties.
Finally, the diselenides can be made into circuits of only three atoms thick, something that is not possible with silicon. The combination of thinner circuits and high-K insulation would allow for the creation of transistors 10 times smaller than anything possible with silicon today.
The two materials—hafnium diselenide and zirconium diselenide— “rust” even better than silicon as they form so-called high-K insulators. Because of this they can be used in thinner layers, enabling lower power operation than is possible with silicon. Furthermore, these ultrathin semiconductors feature a band gap (an energy range in a solid where no electron states can exist) similar to silicon, giving them similar switching properties.
Finally, the diselenides can be made into circuits of only three atoms thick, something that is not possible with silicon. The combination of thinner circuits and high-K insulation would allow for the creation of transistors 10 times smaller than anything possible with silicon today.
Read full article
Hide full article


Discussion (0 comments)