Flow battery can run for 10 years with zero maintenance
February 13, 2017
on
on
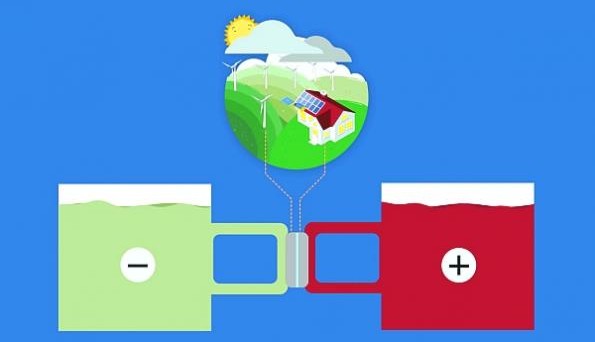
Harvard University researchers have developed a low-cost flow battery that stores energy in organic molecules dissolved in neutral pH water. In their report (see below) they claim that the new battery can run for a decade or more without maintenance.
By modifying the structures of molecules used in the positive and negative electrolyte solutions, and making them water soluble, a battery was produced that loses only one percent of its capacity per 1000 cycles.
The fact that the amount of energy a flow battery is able to store is limited only by the size of the tank makes it “a promising storage solution for renewable, intermittent energy like wind and solar”. Sadly, traditional current flow batteries exhibit degraded energy storage capacity after many charge-discharge cycles, requiring periodic maintenance of the electrolyte to restore the capacity. A low maintenance, long term energy storage system would drastically change the economics of renewable energy.
The key to the technology is to use ferrocene, a molecule well known for its electrochemical properties, for the positive electrolyte. Ferrocene has great charge storage qualities but is completely insoluble in water. The innovation was modifying ferrocene molecules in the same way as viologen, turning an insoluble molecule into a highly soluble one that could also be cycled stably.
Regarding cost, the Department of Energy (DOE) in the US has set a goal of building a battery that can store energy for less than $100 per kilowatt-hour, which would make stored wind and solar energy competitive to energy produced from traditional power plants. The flow battery research is likely to help reach that target soon.
The report is called A Neutral pH Aqueous Organic/Organometallic Redox Flow Battery with Extremely High Capacity Retention.
By modifying the structures of molecules used in the positive and negative electrolyte solutions, and making them water soluble, a battery was produced that loses only one percent of its capacity per 1000 cycles.
The fact that the amount of energy a flow battery is able to store is limited only by the size of the tank makes it “a promising storage solution for renewable, intermittent energy like wind and solar”. Sadly, traditional current flow batteries exhibit degraded energy storage capacity after many charge-discharge cycles, requiring periodic maintenance of the electrolyte to restore the capacity. A low maintenance, long term energy storage system would drastically change the economics of renewable energy.
The key to the technology is to use ferrocene, a molecule well known for its electrochemical properties, for the positive electrolyte. Ferrocene has great charge storage qualities but is completely insoluble in water. The innovation was modifying ferrocene molecules in the same way as viologen, turning an insoluble molecule into a highly soluble one that could also be cycled stably.
Regarding cost, the Department of Energy (DOE) in the US has set a goal of building a battery that can store energy for less than $100 per kilowatt-hour, which would make stored wind and solar energy competitive to energy produced from traditional power plants. The flow battery research is likely to help reach that target soon.
The report is called A Neutral pH Aqueous Organic/Organometallic Redox Flow Battery with Extremely High Capacity Retention.
Read full article
Hide full article


Discussion (0 comments)