KIT develops a single-atom transistor
September 25, 2018
on
on
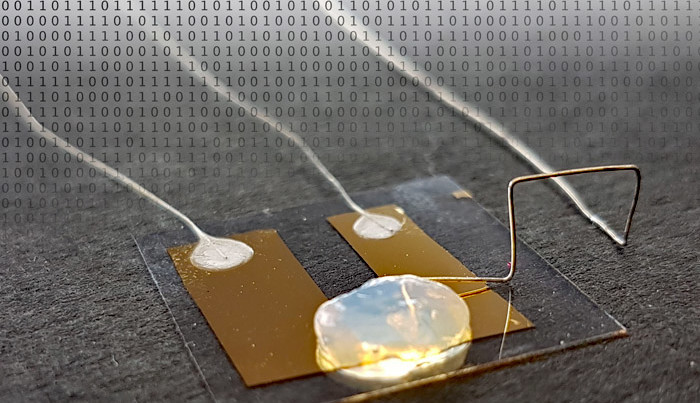
Researchers at the Karlsruhe Institute of Technology (KIT) have demonstrated what is arguably the world's smallest transistor. Developed by Thomas Schimmel and his team, this quantum electronic element uses the displacement of a single atom to switch an electrical path. What’s more, the process works at room temperature!
The development of a single-atom transistor is probably the point where Moore's Law prediction of transistor density will finally hit the buffers. According to our present-day understanding of physics it really will not be possible to make a transistor any smaller. The footprint of such small structures not only benefits from higher integration density but also low energy requirements. This is particularly important because greater transistor density, results in higher energy dissipation in a smaller area and thermal problems. A simple USB memory stick is made up of billions of transistors. This new single-atom transistor requires only about 0.1 % of the energy of a conventional transistor for a switching operation.
In the journal Advanced Materials, the researchers in Karlsruhe describe the transistor design in detail. Between two tiny metal contacts, a gap the width of a single metal atom is left. By means of an electrical control impulse it is possible to push a single silver atom into the gap and thereby close the switch. This single atom can be displaced with another impulse to open the switch. Unlike other quantum electronic devices, however, this single-transistor design does not need to be kept at extremely low temperatures near absolute zero but works at room temperature.
It’s interesting that the transistor is made entirely of metal and doesn’t rely on any semiconducting material to provide current control. This means it only needs a very small control voltage to switch, resulting in extremely low energy consumption. Single-atom transistors designs until now have employed a liquid electrolyte; this new design however uses a solid gel electrolyte, produced by thickening the aqueous silver electrolyte with fumed silica (pyrogenic silicon dioxide). The resulting gel electrolyte combines the advantages of a solid material with the electrochemical properties of a fluid.
The development of a single-atom transistor is probably the point where Moore's Law prediction of transistor density will finally hit the buffers. According to our present-day understanding of physics it really will not be possible to make a transistor any smaller. The footprint of such small structures not only benefits from higher integration density but also low energy requirements. This is particularly important because greater transistor density, results in higher energy dissipation in a smaller area and thermal problems. A simple USB memory stick is made up of billions of transistors. This new single-atom transistor requires only about 0.1 % of the energy of a conventional transistor for a switching operation.
In the journal Advanced Materials, the researchers in Karlsruhe describe the transistor design in detail. Between two tiny metal contacts, a gap the width of a single metal atom is left. By means of an electrical control impulse it is possible to push a single silver atom into the gap and thereby close the switch. This single atom can be displaced with another impulse to open the switch. Unlike other quantum electronic devices, however, this single-transistor design does not need to be kept at extremely low temperatures near absolute zero but works at room temperature.
It’s interesting that the transistor is made entirely of metal and doesn’t rely on any semiconducting material to provide current control. This means it only needs a very small control voltage to switch, resulting in extremely low energy consumption. Single-atom transistors designs until now have employed a liquid electrolyte; this new design however uses a solid gel electrolyte, produced by thickening the aqueous silver electrolyte with fumed silica (pyrogenic silicon dioxide). The resulting gel electrolyte combines the advantages of a solid material with the electrochemical properties of a fluid.
Read full article
Hide full article


Discussion (0 comments)